
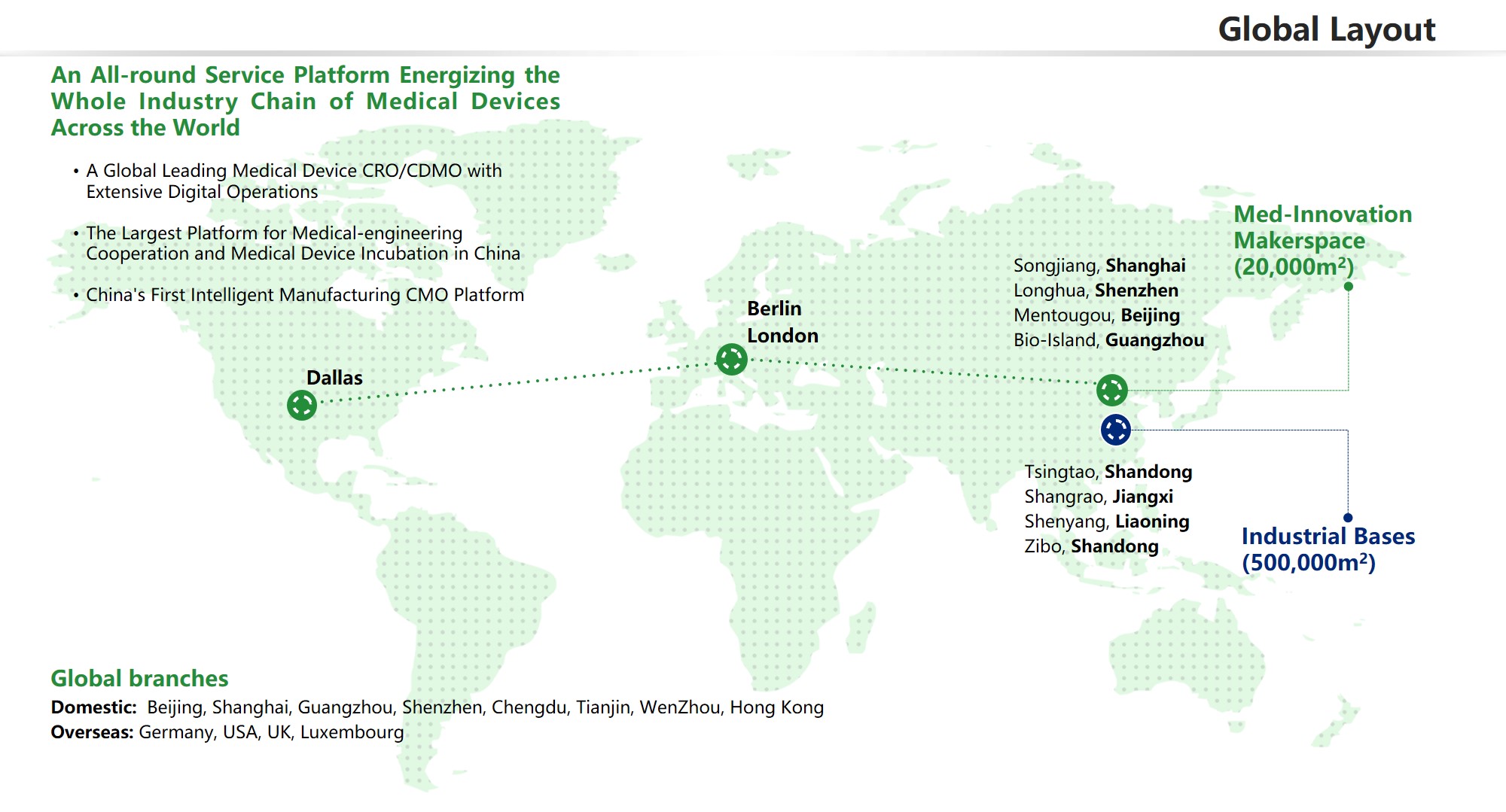
OSMUNDA Medical Device Service Group is an outstanding industry-wide third-party service provider for medical devices across the world (CDMO + CRO + CSO) serving the medical device industry with a medical device Cloud service plarform, a platform of medical device R&D and contract manufacturing, a platform for global registrations and Clinical trials, an investment and financing service platform (patent appraisal, BOC-OSMUNDA Loan) and an industrial service platform (industrial planning, co-building 3C paltform)
Our powerful and professional medical device database, project management system, R&D and contract manufacturing bases (CDMO factories in Beijing, Shangai, Guangzhou and Shenzhen) as well as the 3C industry platform jointly built with the government, guarantee the efficient project operation for our clients.
OSMUNDA has 12 wholly-owned branches worldwide, nine in China (beijing, Shanghai, Guangzhou, Shenzhen, Suzhou. Chengdu, etc.) and four overseas (USA, Germany, UK, Luxembourg). More than 300 medical device experts worldwideare ready to provide global medical device developers, enterprises, and governments with all-around and one-stop medical device industry solutions.
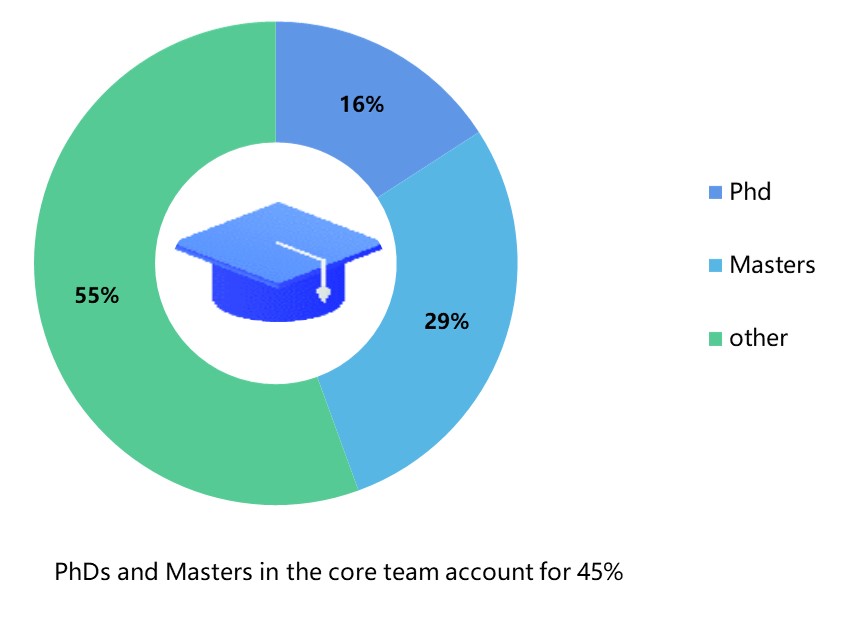
Team Background
● Member of 6 national or local standardization committees
● Engaged in drafting or reviewing Chinese National Standard for 200 times
● 20+ professional instructors of Advanced Study & Training Center of OSMUNDA
Operation Team

● Global Medical Device Strategic Planner
● China/International Medical Device Regulatroy Expert
● Member of the National Medical Device Expert Database
● Top Management of Medical Device Manufacturing
● Medical Device R&D Expert
● Senior Expert in Medical Device Clinical Trials
● Laboratory and Testing Expert
● Multidisciplinary Expert in Clinical Medicine
● Statistical Analysis Expert (Medical Devices/Drugs)
● Data Management Expert (Medical Devices/Drugs)
● Medical Device Industry Data Analyst
● Medical Device Circulation
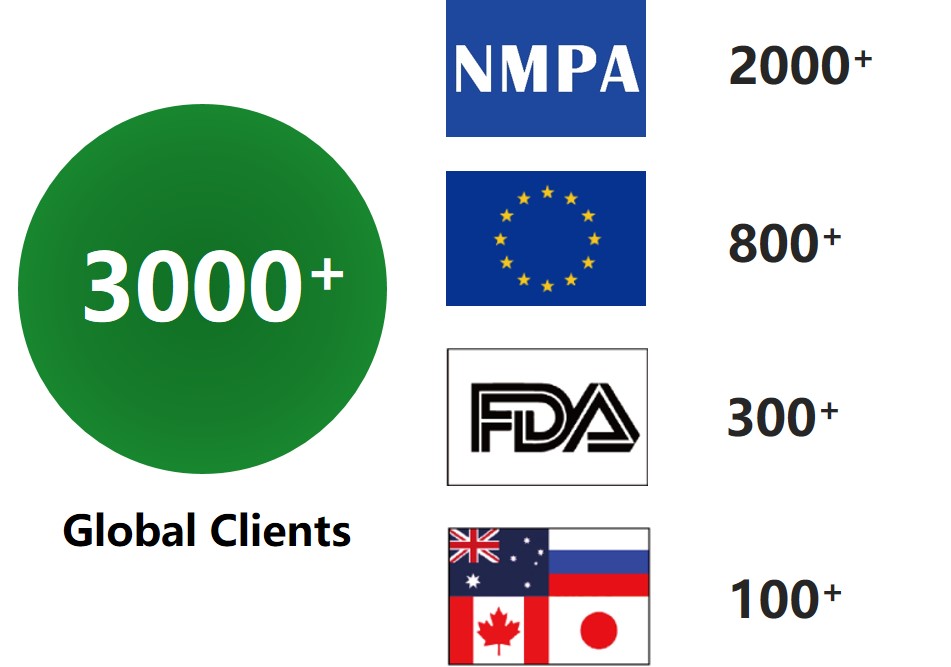
Global Experience
Expertise in High-end Medical Device
Cardiovascular Surgical Devices ![]()
Craniocerebral Surgical ![]()
Ophthalmic Devices ![]()
Orthopedic Instruments ![]()
Oncology Devices ![]()
IVD Reagents ![]()
Comprehensive Application ![]()

 +49 30 8186 5124
+49 30 8186 5124 global@osmundacn.com
global@osmundacn.com
 Osmunda
Osmunda