
Medical device registration and approval in Singapore fall under the purview of the Health Sciences Authority (HSA). The primary regulations governing this process include the Health Products Act 2007 and the Health Products (Medical Devices) Regulations 2010. Additionally, being a member of the ASEAN community, Singapore also considers relevant regulations from ASEAN in its regulatory framework.
For manufacturers outside Singapore, it is mandatory to appoint a License Holder. This agent will be responsible for submitting registration documents, liaising with authorities, and overseeing post-market activities. Registration applications can be submitted online through the Medical Device Information and Communication System (MEDICS). Upon approval, the device is included in the Singapore Medical Device Register (SMDR) database.
Risk classification of medical devices is based on their design and intended use. Singapore categorizes medical devices (including IVDs) into four classes - A, B, C, and D, ranging from low to high risk.
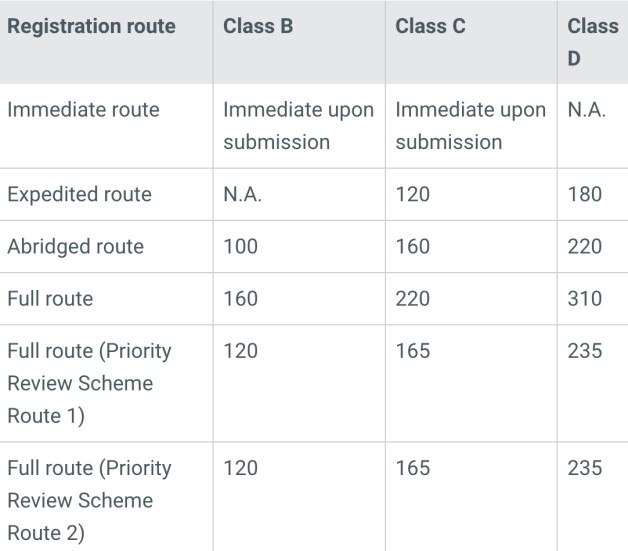
The registration pathways in Singapore are influenced by the classification of the device and its approval status in recognized reference countries such as the United States, Canada, Australia, Japan, and the European Union. There are four registration pathways: Immediate, Expedited, Abridged, and Full. Generally, a smoother and faster registration process is expected for products with approvals in multiple reference countries. In cases of innovative products meeting specific criteria, HSA may offer priority case reviews, accelerating the approval time by approximately 25%.
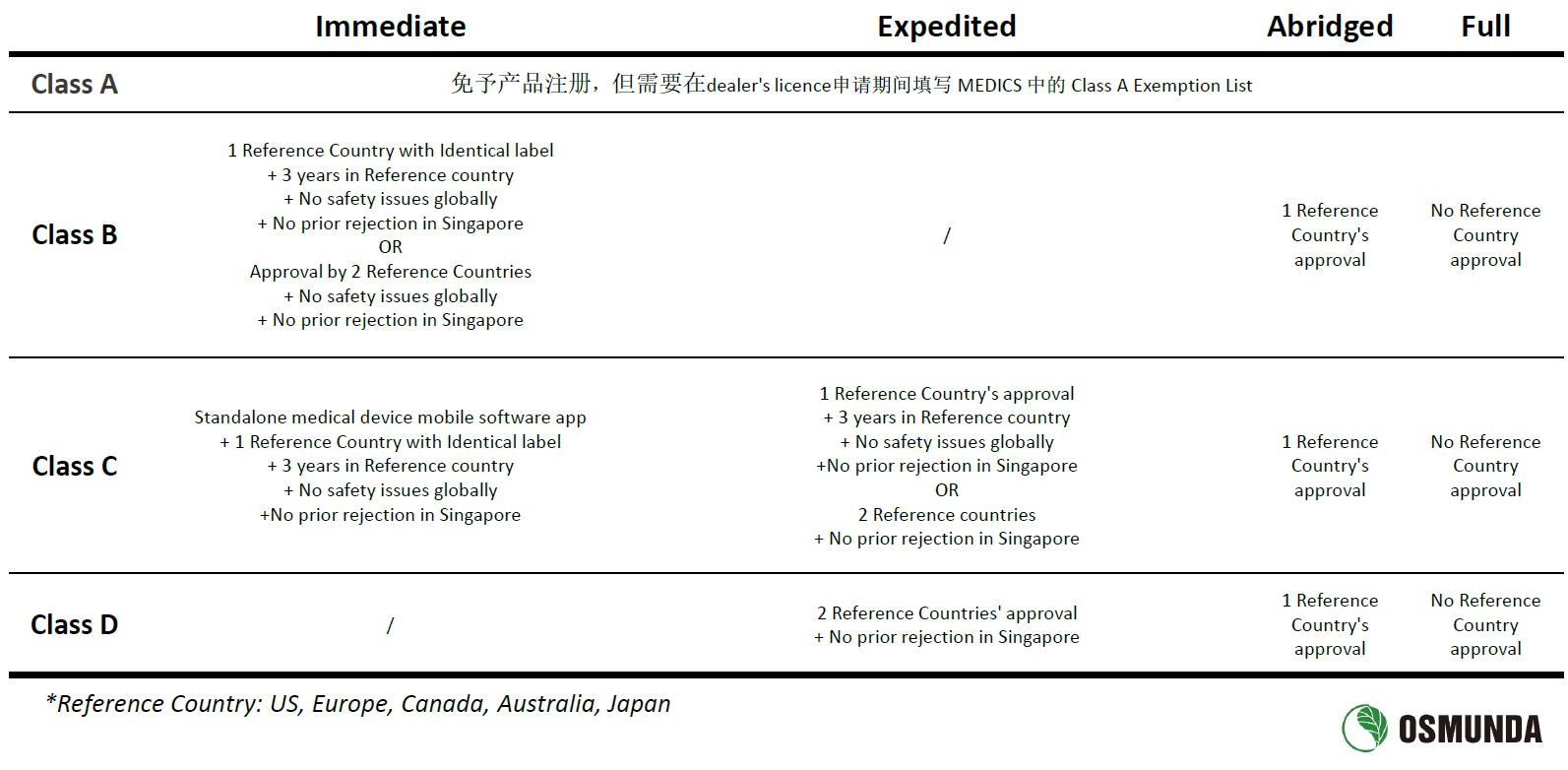
The requirements for technical documents are based on the ASEAN CSDT (Common Submission Dossier Template), aligning with elements such as the Declaration of Conformity, product description, labeling details, risk analysis, Clinical Evaluation Report, and Quality Management System (QMS) related documents. Specific technical documentation requirements vary based on the risk classification and chosen registration pathway.
For the Quality Management System, HSA accepts certifications such as ISO 13485, MDSAP, 21 CFR Part 820 (US FDA), or compliance with the Japanese MHLW Ordinance 169.
The time and cost involved differ depending on the device classification and chosen registration pathway. For instance, Class A products may be exempted from registration, resulting in the shortest processing time and the lowest costs. Conversely, high-risk products may necessitate a registration timeline of 200-300 days, incurring official fees ranging from approximately $10,000 to $20,000 USD, contingent on the chosen registration pathway.
The similar products from the same manufacturer, or products that need to be used together, can be submitted in the form of grouping, thus saving time and cost.
Osmunda can assist you with HSA registration and provide you with local agent service in Singapore. If you would like to know more about the process of your product in Singapore, please feel free to contact us.