

OSMUNDA CDMO Services
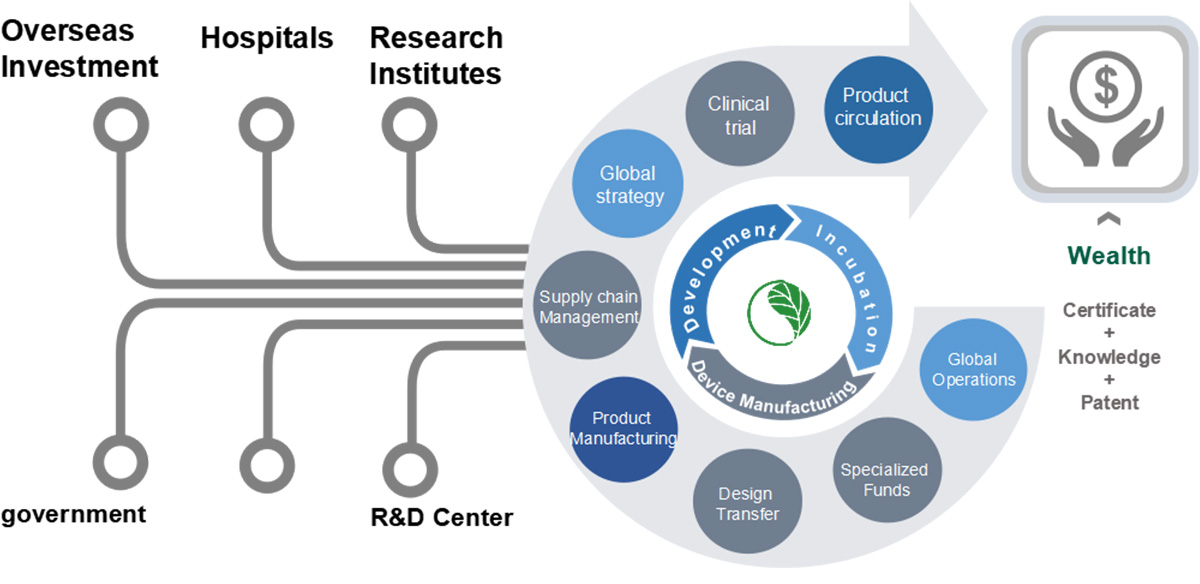
OSMUNDA Medical Device Service Group is an outstanding industry-wide third-party service provider for medical devices across the world (CDMO+CRO+CSO), serving the medical device industry with a medical device Cloud service platform, a platform of medical device R&D and contract manufacturing, a platform of global registrations and clinical trials , an investment and financing service platform (patent appraisal, BOC – OSMUNDA Loan) and an industrial service platform (industrial planning, co-building 3C platform).

Contract Manufacturing
A brilliant idea will remain as an unperceivable illusion if it is not materialized. You can transform your idea into reality with our CDMO full-package services.
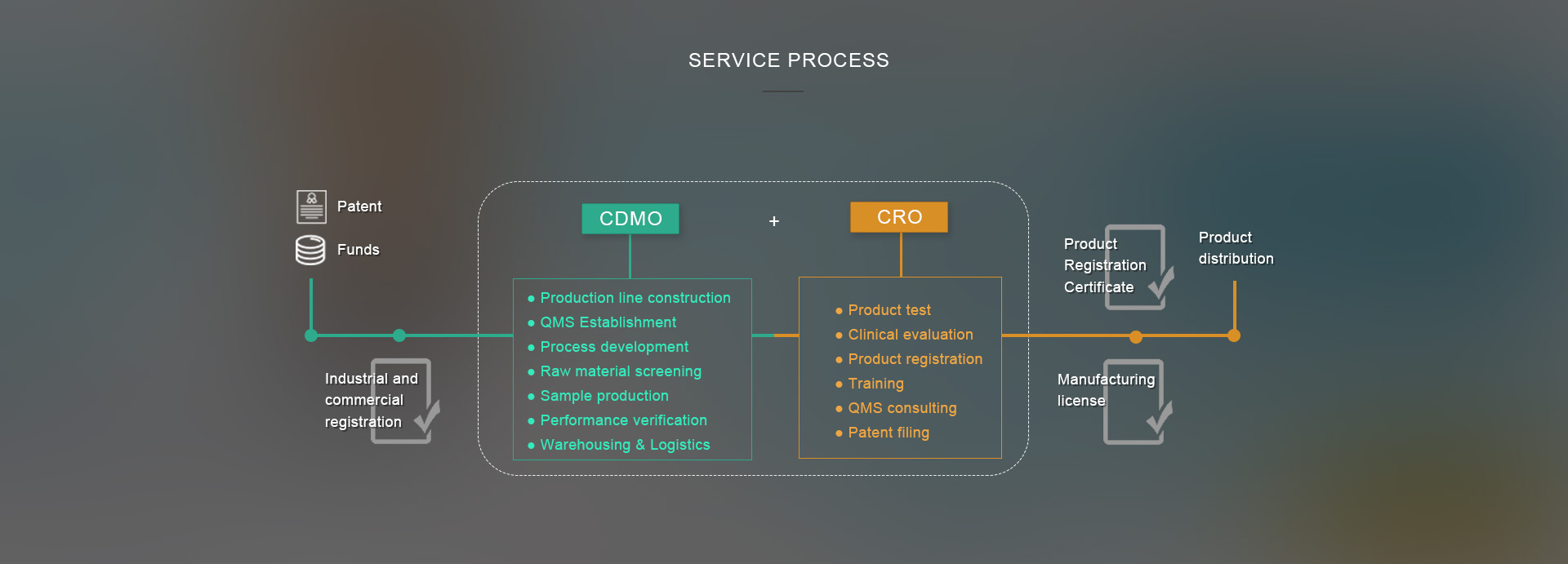
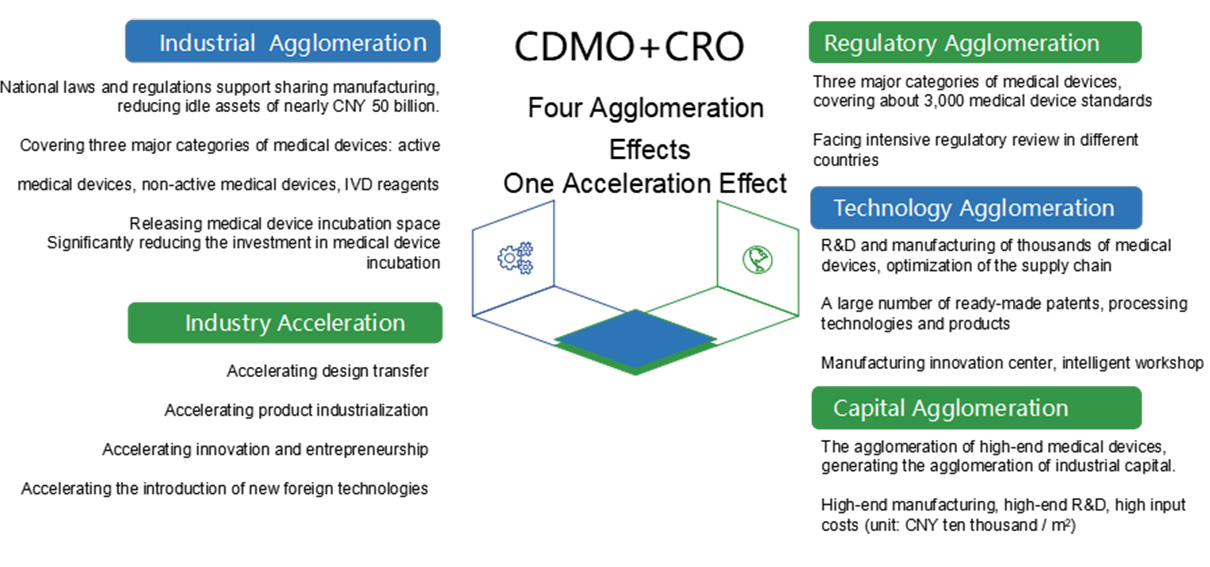
The CDMO platform, an emerging outsourcing service model, achieves the seamless docking with the enterprise’s entire supply chain system (R&D, procurement, production, etc.) from the pre-clinical research, clinical trials to commercial production stage, providing enterprises with innovative process R&D, sample production and large-scale production services.
Predicament of medical device start-ups
2. Daily routine work (e.g. looking for sites, team management, policy study, etc.) is time-consuming, which easily leads to a delay in obtaining the registration certificate.
3. The waiting period for registration application is long, resulting in vacancy and waste of a large number of resources such as workshops, QMS, equipment, personnel, funds, etc.
4. Too many laws and regulations on medical devices lead to a lack of in-depth understanding.
5. The medical device industry is developing rapidly, with an accelerating pace of technological updating.
6. It is difficult to set up a talent team, lack of senior technical talents, and the management cost remains high.
7. The lack of R&D transformation (incubation) capability makes it impossible to realize design transfer.
8. Low R&D concentration, insufficient R&D costs, and short of funds for self-built workshops.
9. Incomplete supply chain management makes risk control impossible.
In response to the above problems faced by medical device start-ups, OSMUNDA, in accordance with the MAH system,
provides targeted one-stop solutions to provide assistance to start-ups:

Our Services:
Sample manufacturing - active MD / non-active MD / IVD
Mass manufacturing - active MD / non-active MD / IVD
Customized manufacturing
Workshop leasing
Production line transfer
Manufacturing solutions based on GMP, ISO 13485, YY/T 0287
Incubation of concepts and ideas
Purchase of patented technology
R&D design and incubation
Industry resource docking
Policy interpretation and guidance
Predicament of medical device supply enterprises:
2. The absence of self-owned brands makes it easy to lose pricing and market initiative.
3. Difficulties and perplexities in business transformation under the risk of ever-changing policies.
4. Profit margins are further compressed due to unified bidding.
5. The medical device industry is developing rapidly, with an accelerating pace of technological updating.
6. Facing the risk of being integrated by large circulation enterprises due to the weak competitiveness of individual entities.
In response to the above challenges faced by medical device supply enterprises, OSMUNDA, in accordance with the MAH system,
provides targeted one-stop solutions to provide assistance to supply enterprises:

Our Services:
Sample manufacturing - active MD / non-active MD / IVD
Mass manufacturing - active MD / non-active MD / IVD
Customized manufacturing
Workshop leasing
Incubation of concepts and ideas
Purchase of patented technology
R&D design and incubation
Industry resource docking
Policy interpretation and guidance

Predicament of scientific research institutes:
2. It takes a long time to obtain the Medical Device Registration Certificate, involving various complicated steps such as workshop establishment, quality management system (QMS), production standards, testing, animal experiments, clinical trials, registration, corrections, etc. Besides, daily routine work (e.g. looking for sites, team management, policy study, etc.) is time-consuming, which easily leads to a delay in obtaining the registration certificate.
3. The waiting period for registration application is long, resulting in vacancy and waste of a large number of resources such as workshops, QMS, equipment, personnel, funds, etc.
4. Too many laws and regulations on medical devices lead to a lack of in-depth understanding.
5. Short of funds for self-built workshops.
In response to the above challenges faced by scientific research institutes, OSMUNDA, in accordance with the MAH system,
provides targeted one-stop solutions to provide assistance to scientific research institutes:
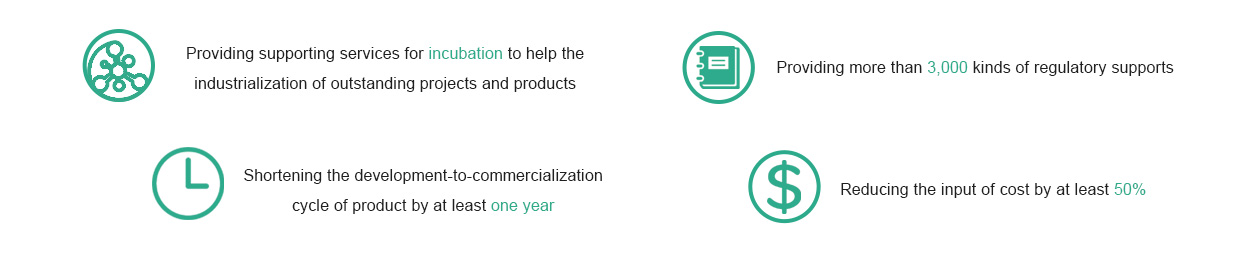
Our Services:
Incubation of scientific research concepts
Product specification definition
Industrial design
Performance and reliability verification
Patented technology transfer
Operating management consulting
Sample manufacturing - active MD / non-active MD / IVD
Mass manufacturing for active MD / non-active MD / IVD
Customized manufacturing
Workshop leasing
Industry resource docking
Financing recommendation for scientific research
Marketing planning of scientific research achievements
Policy interpretation and guidance

Predicament of investment and financing institutions:
2. High cost of trial-and-error.
3. Too many laws and regulations on medical devices lead to a lack of in-depth understanding.
4. Channels for obtaining outstanding projects / products are fragmented.
In response to the above challenges faced by investment and financing institutions, OSMUNDA, in accordance with the MAH system,
provides targeted one-stop solutions to provide assistance to investment and financing institutions:

Our Services:
Industry resource integration
Industry incubation
Talent training and delivery
Project feasibility analysis
Investment and financing analysis and suggestions
Financing and investment recommendation for scientific research
Predicament of doctors/hospitals
2. It takes a long time to obtain the Medical Device Registration Certificate, involving various complicated steps such as workshop establishment, quality management system (QMS), production standards, testing, animal experiments, clinical trials, registration, corrections, etc.
3. Daily routine work (e.g. looking for sites, team management, policy study, etc.) is time-consuming, which easily leads to a delay in obtaining the registration certificate.
4. The waiting period for registration application is long, resulting in vacancy and waste of a large number of resources such as workshops, QMS, equipment, personnel, funds, etc.
5. Too many laws and regulations on medical devices lead to a lack of in-depth understanding.
6. Insufficient R&D costs, and short of funds for self-built workshops.
7. Lack of experience in enterprise operation and management.
In response to the above challenges faced by doctors/hospitals, OSMUNDA, in accordance with the MAH system,
provides targeted one-stop solutions to provide assistance to doctors/hospitals:
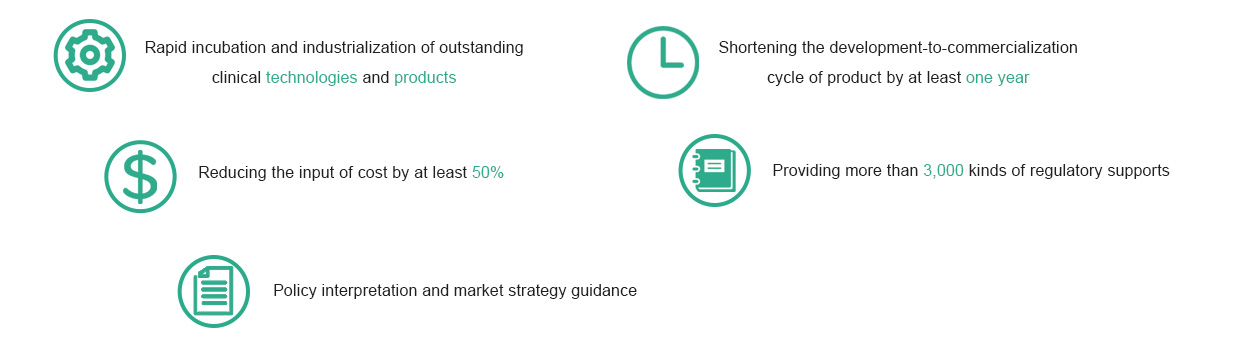
Our Services:
Incubation of technological concepts
Product specification definition
Industrial design
Performance and reliability verification
Patented technology transfer
Operating management consulting
Sample manufacturing - active MD / non-active MD / IVD
Mass manufacturing - active MD / non-active MD / IVD
Customized manufacturing
Industry resource docking
Workshop leasing
Financing recommendation for scientific research
Marketing planning of scientific research achievements
Policy interpretation and guidance


 +49 30 8186 5124
+49 30 8186 5124 global@osmundacn.com
global@osmundacn.com
 Osmunda
Osmunda