
Medical device market - Brazil
Medical Device Registration in Brazil
Medical Device Market in Brazil is expected to reach around $8bn USD in 2023, and maintain an annual growing rate of at least 7% until 2027. However, processes to gain access to the National market are challenging due to the high level of regulation established for these products.
Registration at ANVISA (Brazilian National Health Agency) requires counting with a local representative, QMS and BGMP audits, as well as INMETRO certification (Safety certification, usually required for electro-medical devices subject to IEC 60601, as well as some other medical devices.)
Medical device market - Mexico
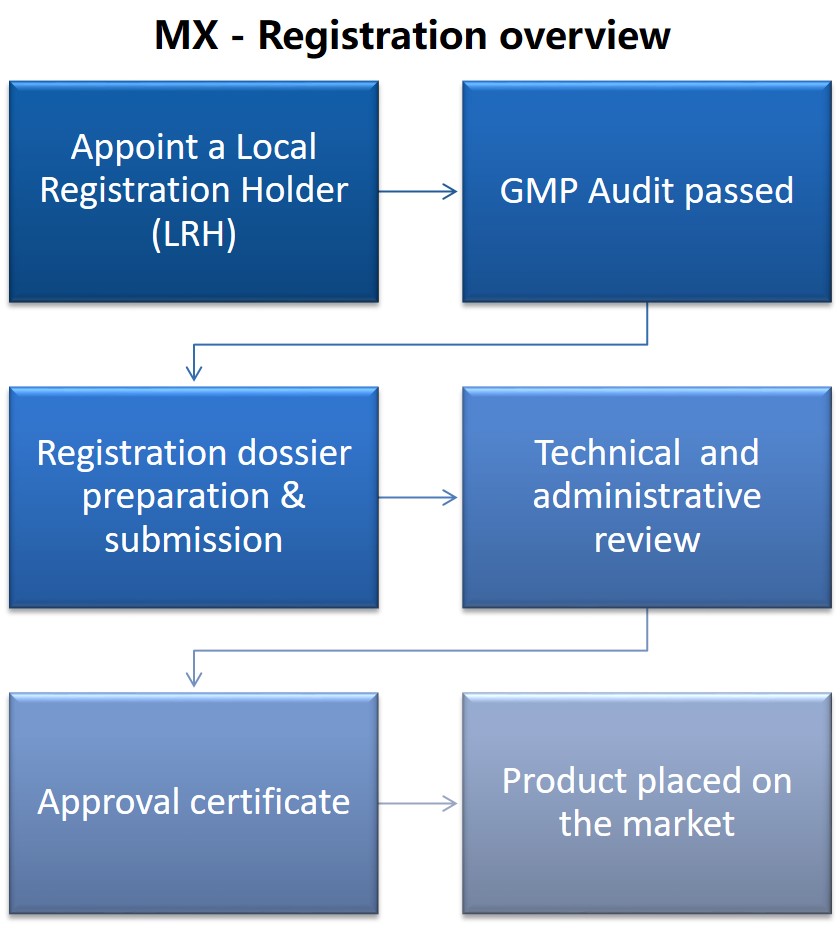
Mexico represents the second medical device market for the Latin America (LATAM) region. The authority responsible for the Medical Device registration and surveillance is COFEPRIS.
Determining the device classification is a crucial step for the registration process, however, national regulations are ambiguous and challenges pertaining to language translations are also prevalent. Good news is clinical and performance studies are not always required to be performed locally.
Medical device market - Peru
The regulatory body for Medical Devices is the Dirección General de Medicamentos, Insumos y Drogas (DIGEMID) under the Ministry of Health (MINSA).
Key Legislation includes:
Law No. 29459: Law of Pharmaceuticals, Medical Devices, and Health Products.
Supreme Decree Nº 016-2013-SA: Regulation for Registration, Control, and Health Surveillance of Pharmaceutical Products, Medical Devices, and Health Products.
Classification of devices is based on risk factors, contact duration, invasiveness, and effects, aligned with IMDRF guidelines.
Manufacturers need to provide the following registration documentation:
Application
Free Sales Certificate (or equivalent)
Good Manufacturing Practice certificate (or equivalent)
Technical Report
Technical and Analytical Studies
Disposal methods (if applicable)
Draft package labeling
Fee
Additional documents based on device class
Timelines and Costs
Class I: 60 days, ~462 USD
Class II: 90 days, ~524 USD
Classes III and IV: 120 days, ~611 USD and ~685 USD respectively
DIGEMID's VUCE (Single Window of Foreign Trade) system facilitates electronic submission and communication, streamlining the regulatory process.
Understanding Peru's medical device regulations and utilizing tools like the VUCE system can help foreign manufacturers efficiently navigate the market and achieve compliance.
Medical device market - Argentina
In Argentina, medical devices are regulated by the National Administration of Drugs, Foods, and Medical Devices (ANMAT). The regulatory framework adheres to the Global Harmonization Task Force (GHTF) guidelines, which are similar to the European Union classification system. Devices are categorized into four classes based on risk: Class I (low risk), Class II (medium risk), Class III (high risk), and Class IV (highest risk).
In vitro diagnostic (IVD) devices are classified separately: Class A represents low risk (e.g., clinical chemistry analyzers), Class B moderate risk (e.g., pregnancy tests), Class C high individual risk (e.g., PSA screening), and Class D high public health risk (e.g., HIV screening).
Labeling for medical devices must include device identification, intended use, instructions for use, contraindications, and warnings, all in Spanish. Higher-class devices also need to provide technical specifications, recognized symbols, sterilization information, and clinical data.
Registrations are valid for five years, with revalidation required 30 days before expiration. Required documentation includes registration forms, a GMP certificate, a CFS, and technical reports, varying by device class. ANMAT requires Certificates of Free Sale (CFS) from recognized countries such as the USA and the EU. Class III and IV devices may require risk analysis, clinical trial results, and other extensive documentation.
The processing time for Class I and II devices is 15 business days, while Class III and IV devices take 110 days.
Fees:
Medical Devices
Class I - ~246 USD
Class II - ~316 USD
Class III - ~420 USD
Class IV - ~586 USD
IVD
Class A & B - ~223 USD
Class C & D - ~276 USD
ANMAT’s regulations ensure that medical devices in Argentina meet international safety and effectiveness standards, facilitating global market access for manufacturers.
Therefore, there is no need to worry, at OSMUNDA, we can support you with every step in the process to successfully place your device in to the Brazilian and Mexican Market.
Interested in Americas markets? Contact us!